About
Digital Diagnostics Website
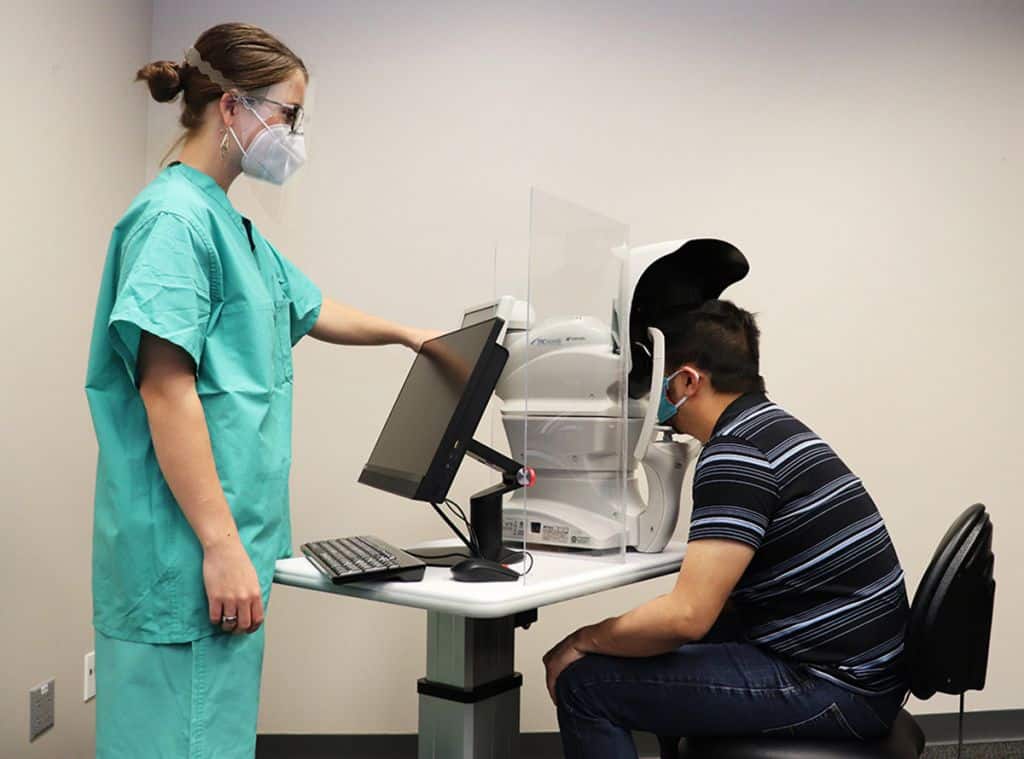
Digital Diagnostics is a pioneering autonomous AI diagnostics company on a mission to transform the quality, accessibility, equity, and affordability of global healthcare through the application of technology in the medical diagnosis and treatment process. Digital Diagnostics’ flagship product, IDx-DR, detects diabetic retinopathy (including macular edema) at point-of-care to provide the patient with a diagnostic report without a physician evaluation. After completing a rigorous prospective, preregistered clinical trial at primary care sites across the country, IDx-DR became the first and only FDA De Novo-cleared AI diagnostic system to make a diagnosis without physician input. IDx-DR is deployed in point-of-care offices across the United States and Europe. The company is paving the way for autonomous AI diagnosis to become a standard of care by working with patient advocacy groups, federal regulators, and other quality of care and ethics-focused stakeholders.
Recent News
Digital Diagnostics and Baxter Announce New Partnership
Digital Diagnostics and Orbis International announce study to help save sight in Bangladesh
Digital Diagnostics Closes $75 million Series B Funding Round led by KKR
8 Questions to Ask When Evaluating an Autonomous AI System that Detects Diabetic Retinopathy
Videos
Founders

Co-Founder and Chief Executive Officer John Bertrand

Co-Founder, President and COO Seth Rainford





