About
Nonagon Website
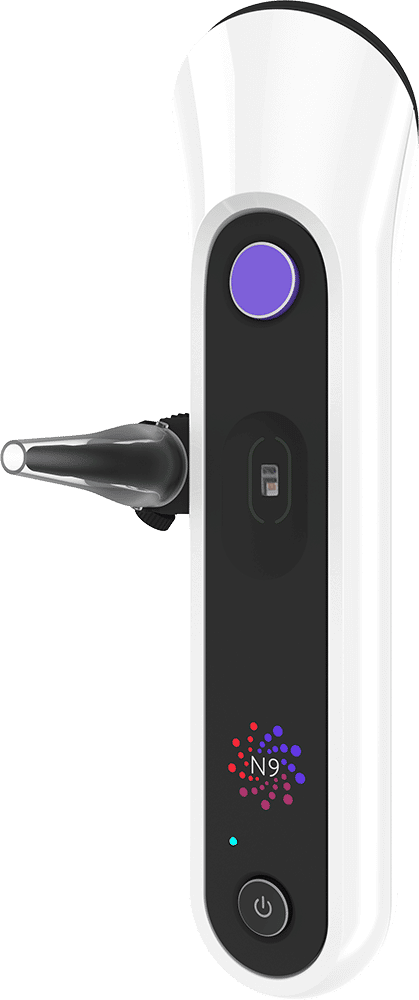
Nonagon’s N9 medical device cleared the FDA in March of 2021. The N9 is a “nine-in-one” clinical grade home device and secure cloud-based software that works with a smartphone and is available by prescription. N9’s nine different functions include a stethoscope, otoscope, pulse oximeter, and temperature monitor, in addition to leveraging the smartphone camera to take photos of the patient’s skin or throat. The patient can use the device on themselves or [e.g.] a child and send the data to the physician before a telemedicine encounter, or it can be used in real-time during a telemedicine visit. The recorded biomarkers include temperature, abdominal sounds, blood oxygen levels, lung sounds, heart sounds, and photos of the inner ear and throat.
Key People
President and CEO Alon Natanson; Chairman of the Board, Josh Ghaim, Ph.D.





